Cellected is launching its new website – in parallel with launching three new, specialist services for iPSC research.
As a number of journal publications make clear – and as recently underlined in the ISSCR Standards document – it is critically important for iPSC researchers to review regularly the quality and stability of their iPSC cultures. Genetic integrity is a key measure of quality, with up to a third of iPSC cultures thought to be affected by genomic or genetic abnormalities. These iPSC genetic or genomic abnormalities cause a substantial adverse downstream impact on experimental and manufacturing reproducibility as well as reliability.
RIQUEST – in-process QC for iPSCs (Rapid, Quality and Stability Testing) helps clients comply with the latest recommendations of the ISSCR Standards as regards QC. (And allows scientists to get on with cutting-edge science – whilst Cellected does the QC.)
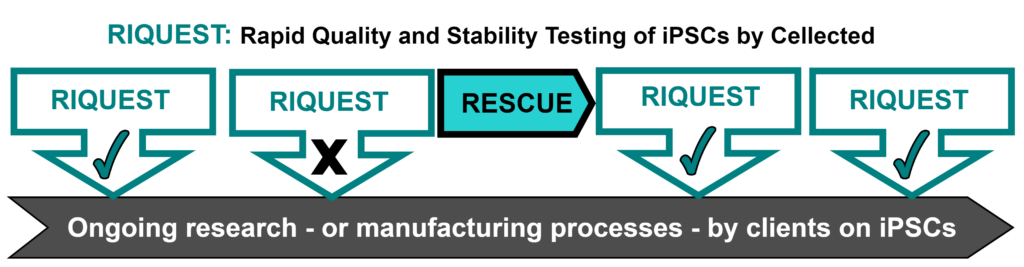
RESCUE – does what it says on the tin – and ‘rescues’ iPSC cell lines which are shown to suffer from genetic or genomic abnormalities. Cellected’s innovative RESCUE service banishes the terror of iPSC QC (and the associated horrors of disposing of cells, and metaphorically research, in the clinical waste bin).
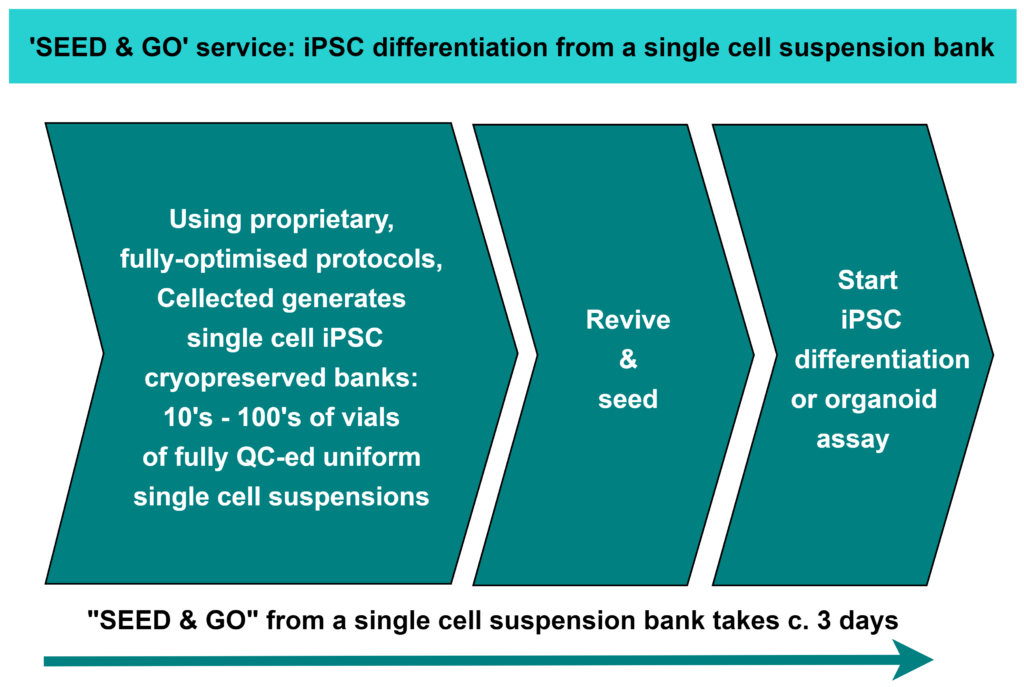
SEED & GO – if you want to reduce the time taken to carry out your iPSC differentiation or organoid assay from some 2 weeks (via the traditional clump-banked approach) to c. 3 days – as well as reduce the variability of results – then ask Cellected about our new results-improving, cost-reducing SEED & GO service.
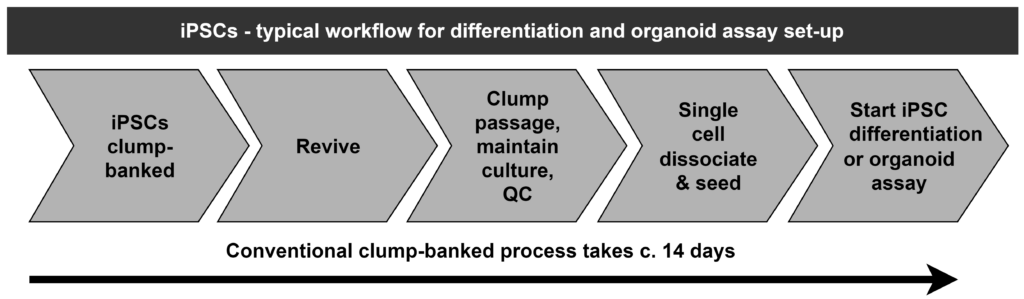
2 weeks versus 3 days. Plus better bio-replicates / reduced variability.