iPSCs are difficult to handle. Reagent selection is important
iPSCs can readily lose their undifferentiated state – and hence lose pluripotency.
Stem cells, especially iPSCs, are difficult to handle and grow up.
The growth of iPSCs will be affected by, among other things, the growth media and the matrix that they are grown in / on as well as the consumables used with the cells.

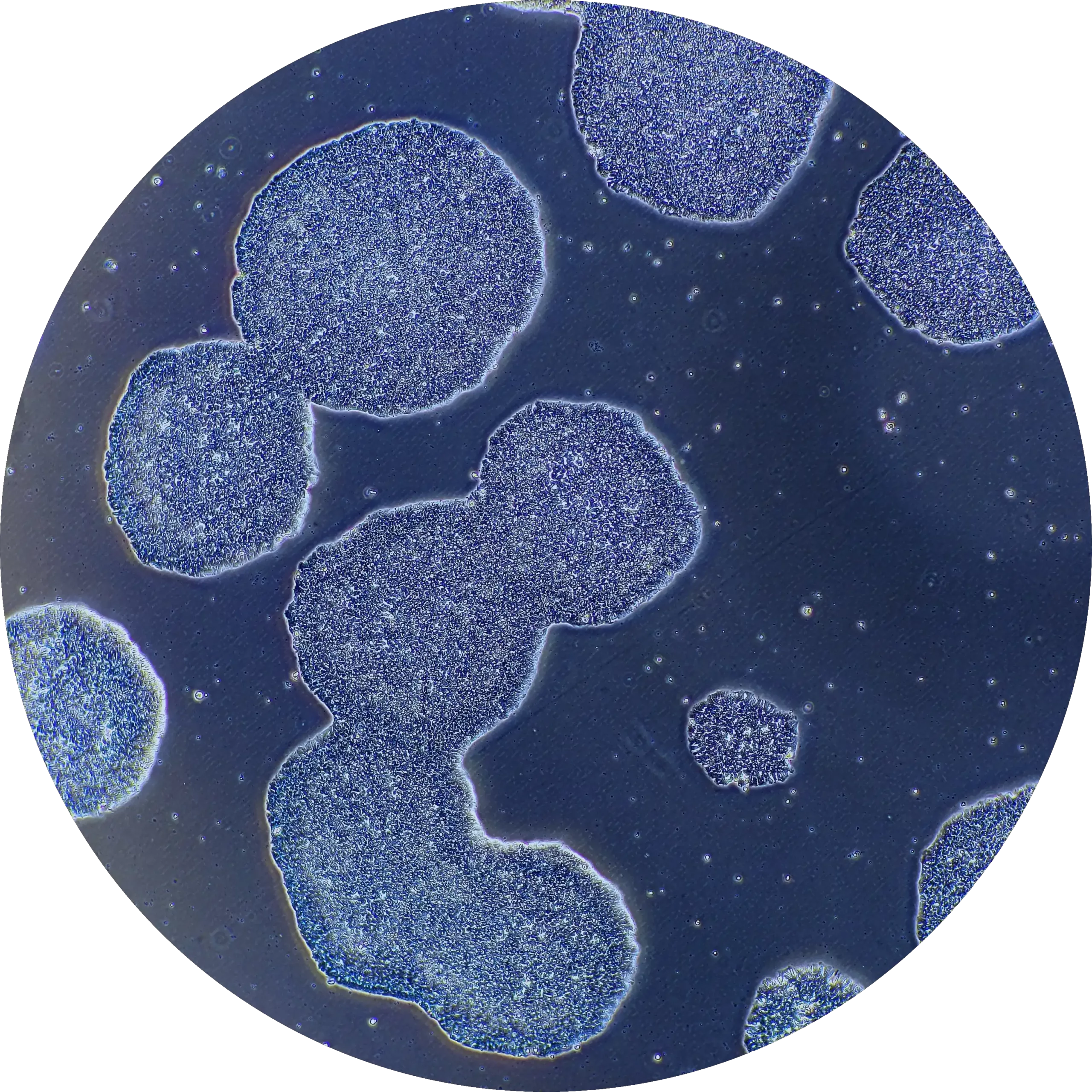
There are real benefits to optimal iPSC reagent selection at the outset of any research programme
Cellected has carried out extensive research work around the different reagents and consumables on the market, including growth media and matrices.
We also help carry out yield optimisation work and reagent selection at the start of a new research programme, mindful of the future requirements of clinical trials / GMP.
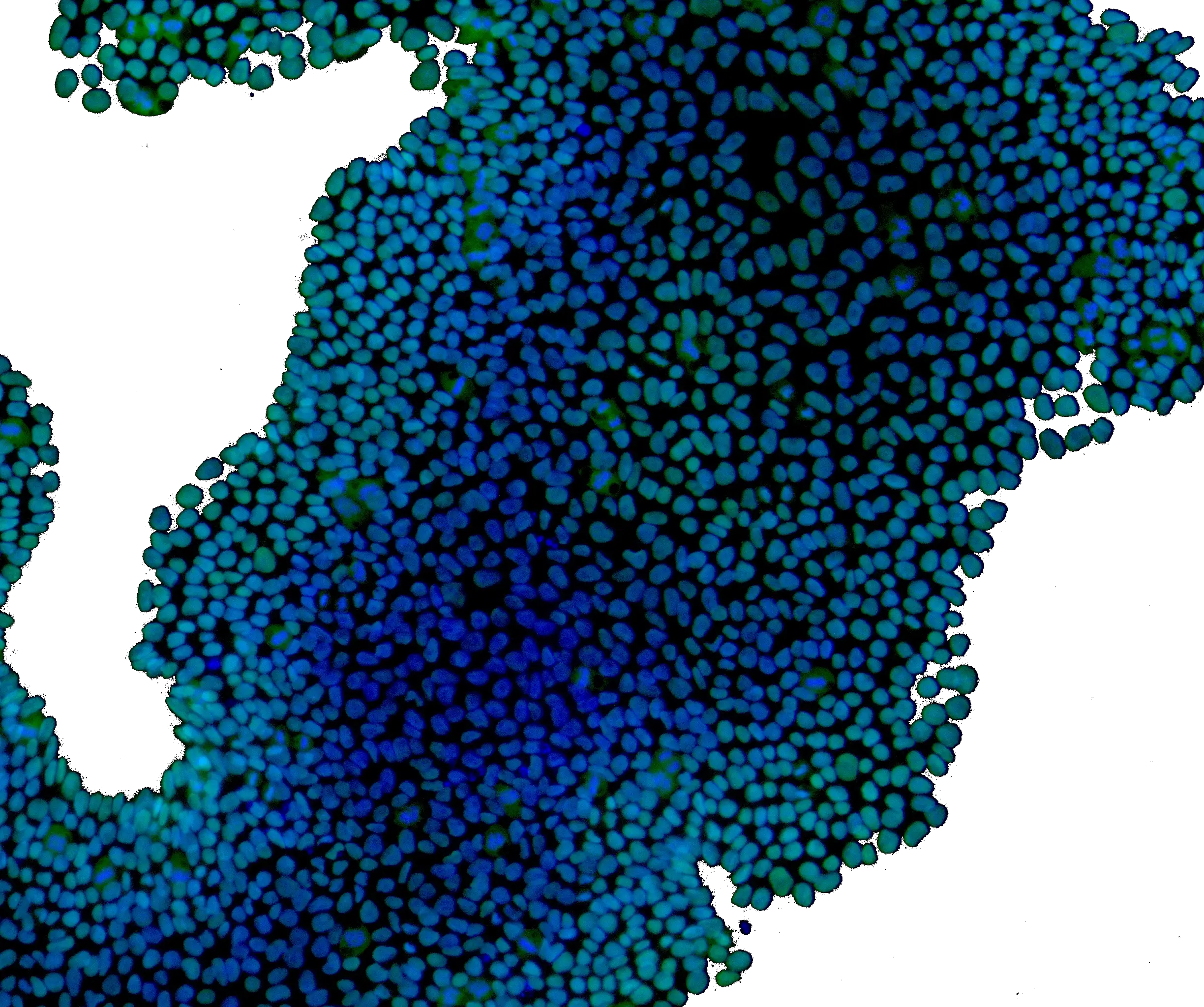
How NOT to select iPSC reagents
As part of our work associated with iPSC reagent and consumables selection, we have seen what not to do on a number of occasions. iPSC reagent selection is challenging – particularly given the interaction between growth media and matrix. Sometimes what not to do is, sadly, quite instructive – see below
A “learning opportunity” on how not to select iPSC reagents
- The research group carries out extensive and successful work – over many months – on a new cell therapy product the work – which initially uses ‘Research Use Only’ (RUO) reagents.
- The data look great. Excitement mounts. Investors are approached.
- The group starts to think about clinical trials… … and then realises that:
- they have been using RUO reagents – often which are not xeno-free – with no ‘off the shelf’ pre-validated, xeno-free clinical grade equivalents; and
- the yield – and hence the ‘Costs of Goods Sold’ (COGS) – is extremely poor / unattractive
- The group then realises that there are substantial costs associated with re-running all the experiments – including the necessary Quality and Stability testing – with optimised, clinical grade reagents for the purposes of generating data for regulatory submissions.
- It becomes painfully clear to all that they should have considered these issues seriously at the beginning of the R&D programme.
So as the ‘learning opportunity’ above illustrates, when working with iPSCs – or indeed any cell line – which one day may, or could, migrate to a clinical trial, make sure that from the outset: (i) you only use RUO reagents that already have a GMP grade equivalent available; and (ii) think about the yield you can obtain early in the process – as if the yield is very poor, then the COGS will be poor – and hence the underlying economics of your clinical product will also be unattractive.
